First malaria treatment for babies approved for use.
In a landmark decision, Swissmedic has approved Coartem Baby—the first-ever malaria treatment specifically designed for newborns under 4.5 kg. Developed by Novartis and Medicines for Malaria Venture, the new formulation fills a life-threatening treatment gap for millions of infants in Africa and other malaria-endemic regions.
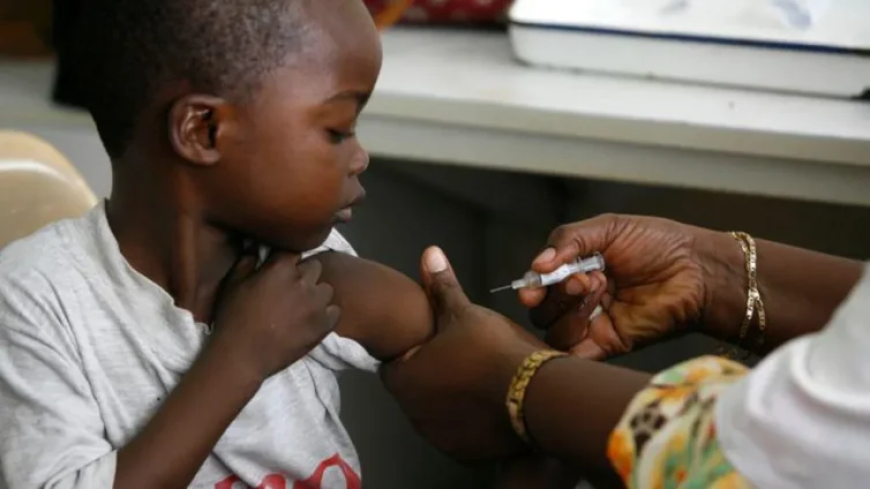
In a major breakthrough for global health, Swiss pharmaceutical regulator Swissmedic has officially approved the first malaria treatment specifically developed for newborn babies under 4.5 kg. The medicine, known as Coartem Baby (or Riamet Baby in some markets), is expected to dramatically reduce infant mortality in malaria-endemic countries, particularly across sub-Saharan Africa.
The drug was developed through a collaborative effort between Novartis and the Medicines for Malaria Venture (MMV) as part of the PAMAfrica consortium. It is the first approved formulation tailored to meet the needs of infants — a group previously neglected in malaria drug development due to their delicate physiology and risk of overdosing from adult or pediatric dosages.
“This is a game-changer in our fight against childhood malaria,” said Martin Fitchet, CEO of MMV. “Newborns have different metabolic profiles and liver function. Until now, healthcare workers had to estimate doses by cutting or dissolving adult tablets — a risky and imprecise practice.”
A Safer, Child-Friendly Formulation
Coartem Baby comes in a cherry-flavored, dissolvable tablet that can easily be mixed into breast milk or water, making it safe and easy to administer to infants as young as a few days old. The drug has been tested in over 120 infants across eight African countries as part of the CALINA clinical trial, which showed strong safety, efficacy, and tolerability results.
“Before this treatment, infants under 5 kg lacked a standardized and safe malaria therapy. We now have a reliable tool to protect our youngest patients,” said Professor Umberto D’Alessandro of the London School of Hygiene & Tropical Medicine.
Addressing a Critical Treatment Gap
Every year, more than 30 million babies are born in malaria-endemic regions. Although the risk of infection is highest in toddlers and pregnant women, studies show that malaria prevalence among infants under six months ranges between 3.4% and 18.4%. Until now, health workers often used scaled-down adult doses, increasing the risk of under-treatment or toxicity.
According to the World Health Organization (WHO), children under the age of five account for over 75% of the nearly 600,000 annual malaria deaths worldwide. The approval of Coartem Baby represents a pivotal shift toward precision treatment in vulnerable age groups.
What’s Next?
The medication will soon be submitted for regulatory approval in at least eight African countries, including Ghana, Nigeria, Tanzania, Kenya, and Uganda, under Swissmedic’s fast-track Global Health Products initiative. Novartis has committed to making the medicine available at no profit in low-income countries, aligning with global efforts to close health equity gaps.
The rollout is expected to begin later this year, in partnership with health ministries, non-profit organizations, and donor agencies.
Conclusion:
The approval of Coartem Baby marks a historic moment in the global battle against malaria. With this medicine, millions of newborns will now have access to life-saving treatment designed specifically for their needs, reinforcing the global commitment to eradicate malaria and protect the most vulnerable.
As nations prepare to integrate the treatment into their public health systems, this breakthrough offers renewed hope for communities long burdened by one of the world’s deadliest diseases.
What's Your Reaction?




















































































